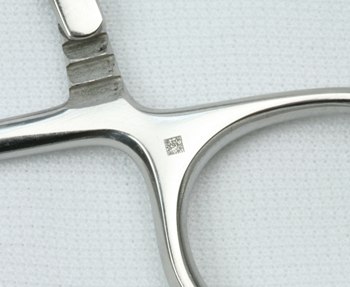
There is a recent release on a UDI ruling regarding the direct marking of the medical device. Device Manufacturers have been implementing data requirements anticipating this ruling and now have a clear understanding of possibly the most important piece; the physical marking on the medical device.
One of the leading factors in the marking process is that the marking is expected to last the lifetime of the device. This can be lengthy as some devices may be reprocessed. In the release the FDA states that there are no specific requirements on the marking, only that all required information is located on the device and that the marking must remain visible during the lifespan of that device.
Manufacturers have invested almost two years implementing the UDI ruling and these medical device marking requirements play a significant role in tracking the medical devices for inventory and in a possible recall.
To view the full article click the link below:
http://www.meddeviceonline.com/doc/fda-clarifies-medical-device-udi-marking-process-0001