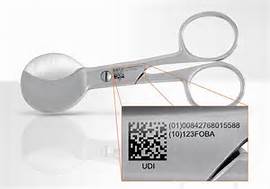
Unique Device Identification – UDI
WHAT IS IT?
The FDA’s Unique Device Identification (UDI) of medical devices aims to offer a practical solution to display standard product information in a standard format for a product’s distribution and use. This information needs to include a UDI in human and machine-readable form and includes (but is not limited to):
- the manufacturer of the device
- expiry dates
- the make and model of the device
- serial or lot number
- any special attributes that the device may possess.
As part of the directive, organizations must submit the product information in a computer-readable structured labeling format to GUDID (Global Unique Device Identification Database) – a publicly searchable database.
UDI is relevant to any organization working in or supplying medical device products (class I, II, and III) to the USA.
A UDI is a unique numeric or alphanumeric code that consists of two parts:
A device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device.
A production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- the lot or batch number within which a device was manufactured;
- the serial number of a specific device;
- the expiration date of a specific device;
- the date a specific device was manufactured;
- the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
As part of the UDI system, the FDA is also creating the Global Unique Device Identification Database (GUDID) which will include a standard set of basic identifying elements for each device with a UDI. Most of this information will be made available to the public so that users of a medical device can easily look up information about the device. The UDI does not indicate, and the database will not contain, any information about who uses a device, including personal privacy information.
The FDA has issued Global Unique Device Identification Database (GUDID) – Guidance for Industry and FDA Staff (pdf) to give labelers an overview of the GUDID. This guidance is designed to help labelers prepare to submit information to the GUDID by describing key GUDID concepts such as accounts, user roles, the device identifier record life cycle, package configurations, and the GUDID data attributes and descriptions.
Benefits of Unique Device Identification
When fully implemented, the UDI system can:
- Allow more accurate reporting, reviewing, and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
- Reduce medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
- Enhance analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources, and registries. A more robust post-market surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
- Provide a standardized identifier that will allow manufacturers, distributors, and healthcare facilities to more effectively manage medical device recalls.
- Provide a foundation for a global, secure distribution chain, helping to address counterfeiting and diversion and prepare for medical emergencies.
In 2013, the Food and Drug Administration (FDA) released a final rule establishing a unique device identification system designed to adequately identify devices through distribution and use. The final rule requires device labelers to include a unique device identifier (UDI) on device labels and packages, except where the rule provides for an exception or alternative. Each UDI must be provided in a plain-text version and in a form that uses automatic identification and data capture (AIDC) technology. The UDI will also be required to be directly marked on a device that is intended for more than one use and intended to be reprocessed before each use. Dates on device labels and packages are to be presented in a standard format that is consistent with international standards and international practice.
A UDI is a unique numeric or alphanumeric code that consists of two parts:
a device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device, and
a production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
the lot or batch number within which a device was manufactured;
the serial number of a specific device;
the expiration date of a specific device;
the date a specific device was manufactured;
the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
All UDIs are to be issued under a system operated by an FDA-accredited issuing agency. The rule provides a process through which an applicant would seek FDA accreditation, specifies the information that the applicant must provide to FDA, and the criteria FDA will apply in evaluating applications.
Certain exceptions and alternatives are outlined in the final rule, ensuring that the costs and burdens are kept to a minimum. The UDI system will go into effect in stages, over a period of seven years, to ensure a smooth implementation and to spread the costs and burdens of implementation over time, rather than having to be absorbed all at once.
As part of the system, the device labelers are required to submit information to the FDA-administered Global Unique Device Identification Database (GUDID). The GUDID will include a standard set of basic identifying elements for each device with a UDI, and contain ONLY the DI, which would serve as the key to obtain device information in the database. PIs are not part of the GUDID.
FDA is making most of this information available to the public at AccessGUDID, through a partnership with the National Library of Medicine. Users of medical devices can use AccessGUDID to search or download information about devices. The UDI does not indicate, and the GUDID database will not contain, any information about who uses a device, including personal privacy information.
For more information on the GUDID and UDI please see the UDI Resources page where you’ll find links to helpful education modules, guidances, and other UDI-related materials.
A “labeler” is any person who causes a label to be applied to a device, or who causes the label of a device to be modified, with the intent that the device will be commercially distributed without any subsequent replacement or modification of the label. The addition of the name of, and contact information for, a person who distributes the device, without making any other changes to the label is not a modification for the purposes of determining whether a person is a labeler. In most instances, the labeler would be the device manufacturer, but the labeler may be a specification developer, a single-use device reprocessor, a convenience kit assembler, a repackager, or a relabeler.
Automatic identification and data capture (AIDC) means any technology that conveys the UDI or the device identifier of a device in a form that can be entered into an electronic patient record or another computer system via an automated process.