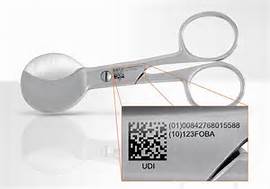
The FDA established the Unique Identification System (UDI) to identify medical devices sold in the US from the point of manufacturing and distribution all the way to patient use.
The UDI final rule requires device labelers to:
- Include a UDI on device labels and packages, except where the rule provides for an exception or alternative.
- Submit device information to the Global Unique Device Identification Database (GUDID).
The benefit of this rule is to easily identify medical devices for safe and effective use which would reduce medical errors that result from the misidentification of a device or confusion about appropriate use. The rule also required dates on the device labels to conform to a standard format to ensure those dates are clearly understood by the device user.
While the ruling was established in 2013, the requirements were designed to be phased in over seven years based on a device’s classification. Immediate action and compliance were required for devices under the classification of implantable, life-supporting, and life-sustaining. The timeline for UDI labeling of devices not under these classifications has been extended from September 2020 to September 2022.
Pragmatyxs ensures all required data measures are implemented when providing labeling solutions for our clients. Whether we are handling label design creation or implementing a new or updated software solution, the UDI regulations are upheld. Make sure you have a Trusted Advisor to assist with your medical device labeling needs.